Metals
Aluminum (Al/13)
Description: Aluminum or aluminium is a silver-white metal, very light in weight (less than three times as dense as water), yet relatively strong. Because aluminum is ductile, it can be drawn into wires or pressed into sheets or foil. It is the most abundant metallic element, and the third most abundant of all elements in the Earth's crust, making up 8% of the crust by weight. Only silicon and oxygen are more plentiful. Bauxite ore (see attched image) is the main source of aluminum; bauxite is processed into alumina before being processed into metallic applications. As a general rule, 4 tons of dried bauxite is required to produce 2 tons of alumina, which, in turn, produces 1 ton of aluminum.
Uses: transportation; containers and packaging; building and construction; electrical; machinery and equipment; structural airframe material for aircraft; military and combat vehicles
US Imports: 3,600,000 mt (2022 est.) The US is more than 75% import reliant for its bauxite needs.
Import Sources (2018-21): Bauxite: Jamaica, 63%; Brazil, 9%; Guyana and Turkey, 8% each; and other, 12%
Alumina: Brazil, 59%; Australia and Jamaica, 14% each; Canada, 5%; and other, 8%
World Resources: Global resources of bauxite are estimated to be between 55 to 75 billion tons and are sufficient to meet world demand for metal well into the future.
Substitutions: Composites can substitute for aluminum in aircraft fuselages and wings. Glass, paper, plastics, and steel can substitute for aluminum in packaging. Composites, magnesium, steel, and titanium can substitute for aluminum in ground transportation uses. Composites, steel, vinyl, and wood can substitute for aluminum in construction. Copper can replace aluminum in electrical and heat-exchange applications.
Antimony (Sb/51)
Description: Antimony is a silvery-gray, brittle semi-metal. It rarely occurs in nature as a native element (see attached image), but is found in a number of different minerals, the most important of which is stibnite (see attached image). Antimony is often called a semi-metal because in pure form it is not shiny and malleable like true metals.
Uses: automotive batteries (lead-acid); ceramics and glass; flame retardants (flameproof fabrics); automotive brake pads (additive to adjust co-efficient of friction); cable sheathing
US Imports: 25,590 mt (2022 est.) The US is 83% import reliant for its antimony needs.
Import Sources (2018-21): Ore and concentrates: China, 46%; Italy, 34%; India, 12%; Belgium, 5%; and other, 3%; total metal and oxide: China, 63%; Belgium, 8%; India, 7%; and other, 22%
World Resources: Principal identified world resources are in Australia, Bolivia, China, Mexico, Russia, South Africa, and Tajikistan.
Substitutions: Selected organic compounds and hydrated aluminum oxide are substitutes as flame retardants. Chromium, tin, titanium, zinc, and zirconium compounds substitute for antimony chemicals in enamels, paint, and pigments. Combinations of calcium, copper, selenium, sulfur, and tin are substitutes for alloys in lead-acid batteries.
Note(s): US resources of antimony are mainly in Alaska, Idaho, Montana, and Nevada.
Beryllium (Be/4)
Description: Beryllium is the 44th most abundant element in the earth’s crust. Beryl (see attached image) is a mineral composed of beryllium aluminium silicate. Beryllium is a silvery-white, hard and brittle, extremely light metal, which is highly toxic. The mechanical and thermal properties relating to its low density are superior to those of all other materials, making it very useful for structural and electronic applications. Beryllium metal can be vacuum cast as an ingot or hot pressed as a powder.
Uses: battery contacts and electronic connectors; windows for X-ray tubes; aerospace castings; high-definition and cable television; underwater fiber-optic cable systems; high-density circuits for high-speed computers and automotive ignition systems; pacemakers and other medical devices
US Imports: 41 mt (2022 est.)
Import Sources (2018-21): Kazakhstan, 43%; Japan, 15%; Latvia, 15%; Brazil, 10%; and other, 17%
World Resources: The world’s identified resources of beryllium have been estimated to be more than 100,000 tons. About 60% of these resources are in the US; by size, the Spor Mountain area in Utah, the McCullough Butte area in Nevada, the Black Hills area in South Dakota, the Sierra Blanca area in Texas, the Seward Peninsula in Alaska, and the Gold Hill area in Utah account for most of the total.
Substitutes: Because the cost of beryllium is high compared with that of other materials, it is used in applications in which its properties are crucial. In some applications, certain metal matrix or organic composites, high-strength grades of aluminum, pyrolytic graphite, silicon carbide, steel, or titanium may be substituted for beryllium metal or beryllium composites. Copper alloys containing nickel and silicon, tin, titanium, or other alloying elements or phosphor bronze alloys (copper-tin-phosphorus) may be substituted for beryllium-copper alloys, but these substitutions can result in substantially reduced performance. Aluminum nitride or boron nitride may be substituted for beryllium oxide.
Note(s): US domestic beryllium consumption in 2022 was estimated to be about 180 mt.
Bismuth (Bi/83)
Description: Bismuth is a silvery-white metallic element with a pinkish tint. Bismuth was long thought to be a variety of lead or tin, which it resembles, until the chemist Claude Geoffroy showed in 1753 that it is a separate element. Among the heavy metals, it is the heaviest and the only non-toxic. The most important ores of bismuth are bismuthinite (see attached image) and bismite (see attached image). Native bismuth is known from Australia, Bolivia, and China.
Uses: cosmetics (bismuth oxychloride); pharmaceuticals (compounds used in over-the-counter to treat stomach illness, burns, intestinal disorders, and stomach ulcers); metal alloys; solder; thermoelectric devices (bismuth telluride); fireworks; plastics with opacity to X-rays (implanted medical devices); ammunition (replacement for lead shot used for hunting, "less-lethal" riot projectile)
US Imports: 2,800 mt (2022 est.) The US is 96% import reliant for its bismuth needs.
Import Sources (2018-21): : China, 65%; Republic of Korea, 19%; Mexico, 5%; Belgium, 3%; and other, 8%
World Resources: World reserves of bismuth are usually estimated based on the bismuth content of lead resources because bismuth production is most often a byproduct of processing lead ores. In China and Vietnam, bismuth production is a byproduct or coproduct of tungsten and other metal ore processing. In Japan and the Republic of Korea, bismuth production is a byproduct or coproduct of zinc ore processing.
Substitutes: Bismuth compounds can be replaced in pharmaceutical applications by alumina, antibiotics, and magnesia. Titanium dioxide-coated mica flakes and fish-scale extracts are substitutes in pigment uses. Indium can replace bismuth in low-temperature solders. Resins can replace bismuth alloys for holding metal shapes during machining, and glycerine-filled glass bulbs can replace bismuth alloys in triggering devices for fire sprinklers. Free machining alloys can contain lead, selenium, or tellurium as a replacement for bismuth.
Note(s): Bismuth, at an estimated 8 parts per billion by weight, ranks 69th in elemental abundance in the Earth’s crust and is about twice as abundant as gold. Bismuth minerals rarely occur in sufficient quantities to be mined as principal products; a mine in China is the only one where bismuth is the primary product.
Cadmium (Cd/48)
Description: Cadmium is a very soft, silvery-white metallic element; it is so soft that it can be cut with a knife. Cadmium has many chemical similarities to zinc, but is less reactive with acids than is zinc. Metallic cadmium is rarely used industrially in pure form. Cadmium is generally recovered as a byproduct from zinc concentrates. The cadmium mineral, greenockite (see attached image), is frequently associated with weathered sphalerites and wurtzites, but usually at microscopic levels.
Uses: nickel cadmium (NiCd) batteries; pigments (yellow, orange, and red); plating (provides better rust resistance than zinc, especially in salt water environments)
US Imports: 57 mt (2022 est.) The US is less than 25% import reliant for its cadmium needs.
Import Sources (2018-21): Australia, 27%; Germany, 23%; China, 20%; Peru, 12%; and other, 18%
World Resources: Most of the world’s primary cadmium metal is produced in Asia, and leading global producers are China and the Republic of Korea, followed by Japan and Canada. A smaller amount of secondary cadmium metal is recovered from recycling NiCd batteries.
Substitutes: Lithium-ion and nickel-metal hydride batteries can replace NiCd batteries in many applications. Except where the surface characteristics of a coating are critical (for example, fasteners for aircraft), coatings of zinc, zinc-nickel, or vapor-deposited aluminum can be substituted for cadmium in many plating applications. Cerium sulfide is used as a replacement for cadmium pigments, mostly in plastics. Barium-zinc or calcium-zinc stabilizers can replace barium-cadmium stabilizers in flexible PVC applications. Amorphous silicon and copper-indium-gallium-selenide photovoltaic cells compete with cadmium telluride in the thin-film solar-cell market.
Note(s): Cadmium is generally recovered from zinc ores and concentrates. Sphalerite, the most economically significant zinc ore mineral, commonly contains minor amounts of cadmium, which shares certain similar chemical properties with zinc and often substitutes for zinc in the sphalerite crystal lattice. The cadmium mineral greenockite is frequently associated with weathered sphalerite and wurtzite.
Cesium (Cs/55)
Description: Cesium is a very soft, ductile, alkali metal that is liquid at 28.4° C. It is the most electropositive and reactive of the alkali metals and forms compounds with a variety of anions and alloys with the other alkali metals and with gold. The metal ignites spontaneously in the presence of air and reacts explosively in water. Because of this reactivity, cesium is classed as a hazardous material and must be stored and transported in isolation from possible reactants. Caesium is mined mostly from pollucite (see attached image).
Uses: The current application that likely requires the most cesium is as a specialty high-density component in drilling mud used for petroleum exploration. Cesium also has a wide-spectrum of photoemissive properties whereby electromagnetic radiation, which includes visible light and nearby regions of the radiation spectrum, are converted to electrical current. Thus, cesium is used in television image devices, night-vision equipment, solar photovoltaic cells, and other types of photoelectric cells. Perhaps one of its best known applications is its use in the super-accurate atomic cesium clock that is used as a standard for the world’s timekeeping systems. It is also used in the chemical process industry, primarily as an ingredient of metal-ion catalysts; in medical applications; in the removal of sulfur from crude oil in petroleum refining; and as an ingredient in specialty glasses used in fiber optics and night-vision devices.
US imports: Only a few thousand kilograms of cesium are consumed in the US every year. The US is 100% import reliant for its cesium needs.
Import Sources (2018-21): No reliable data has been available to determine the source of cesium ore imported by the US since 1988. Prior to 2016, Canada was thought to be the primary supplier of cesium ore and refined chemicals. Based on recent import data, it is thought that Germany was a source of refined cesium chemicals.
World Resources: US and world resources of cesium have not been estimated. It is a relatively uncommon element that can be mined in only a few places in the world. During 2021, no primary cesium mine production was reported globally but cesium was thought to have been mined in China. Mine production of cesium from all countries, excluding China, ceased within the past two decades. No reliable data are available to determine reserves for specific countries; however, Australia, Canada, China, and Namibia were thought to have reserves totaling less than 200,000 tons. Existing stockpiles at multiple former mine sites have continued feeding downstream refineries, though recent reports have indicated stockpiles will be depleted within a few years.
Substitutes: Cesium and rubidium can be used interchangeably in many applications because they have similar physical properties and atomic radii. Cesium, however, is more electropositive than rubidium, making it a preferred material for some applications. However, rubidium is mined from similar deposits, in relatively smaller quantities, as a byproduct of cesium production in pegmatites and as a byproduct of lithium production from lepidolite (hard-rock) mining and processing, making it no more readily available than cesium.
Note(s): In 2021, no cesium was mined domestically in the US.
Chromium (Cr/24)
Description: Chromium is a steely-gray, lustrous, hard and brittle metal that takes a high polish, resists tarnishing, and has a high melting point. Chromium is produced from chromite ore (see attached image). About 80% of world production of chromite ore comes from India, Kazakhstan, and South Africa.
Uses: component in nickel super-alloys for land based turbines and jet engines; component in high-speed tool steel; surface coatings; catalysts for processing hydrocarbons; refractory materials; resistance heating wires
US Imports: 620,000 mt (2022 est.) The US is 83% import reliant for its chromium needs.
Import Sources (2018-21): Chromite (mineral): South Africa, 97%; Canada, 2%; and other, 1%; Chromium-containing scrap: Canada, 48%; Mexico, 42%; Netherlands, 4%; and other, 6%; Chromium (primary metal): South Africa, 30%; Kazakhstan, 13%; Russia, 9%; Germany, 7%; and other, 41%; Total imports: South Africa, 37%; Kazakhstan, 10%; Russia, 7%; Germany, 6%; and other, 40%
World Resources: World resources are greater than 12 billion tons of shipping-grade chromite, sufficient to meet conceivable demand for centuries. The world’s chromium resources are heavily geographically concentrated (95%) in Kazakhstan and southern Africa; US chromium resources are mostly in the Stillwater Complex in Montana. South Africa was the leading chromite ore producer. Global chromite ore mine production was estimated to have decreased slightly in 2022, owing to constraints from operational challenges in some countries, slow global economic growth, and increasing labor costs.
Substitutes: Chromium has no substitute in stainless steel, the leading end use, or in superalloys, the major strategic end use. Chromium-containing scrap can substitute for ferrochromium in some metallurgical uses.
Note(s): Stainless steels and superalloys require chromium. In 2022, the United States consumed an estimated 5% of world chromite ore production in various forms of imported materials, such as chromite ore, chromium chemicals, chromium ferroalloys, chromium metal, and stainless steel.
Cobalt (Co/27)
Description: Cobalt is a bluish-gray, shiny, brittle metallic element. It has magnetic properties like iron. Cobalt-nickel alloys have good temperature stability and corrosion and wear resistance and are used in high temperature applications. The cobalt resources identified in the world are mostly found in copper or nickel mines in Australia, Canada, Democratic Republic of the Congo (DROC), Russia, and Zambia. In the US, cobalt resources are in mostly found in Minnesota. Most of the cobalt used in the US is imported. An estimated 42% of the cobalt consumed in the United States was used in superalloys, mainly in aircraft gas turbine engines; 9% in cemented carbides for cutting and wear-resistant applications; 16% in various other metallic applications; and 33% in a variety of chemical applications.
Uses: batteries; component in nickel superalloys for high temperature sections of jet engines and industrial gas turbines; pigments; medical implants
US Imports: 11,000 mt (2022 est.) The US is 76% import reliant for its cobalt needs.
Import Sources (2018-21): Cobalt contained in metal, oxide, and salts: Norway, 22%; Canada, 16%; Finland, 12%; Japan, 12%; and other, 38%
World Resources: Identified world terrestrial cobalt resources are about 25 million tons. The vast majority of these resources are in sediment-hosted stratiform copper deposits in DROC and Zambia; nickel-bearing laterite deposits in Australia and nearby island countries, and in Cuba; and magmatic nickel-copper sulfide deposits hosted in mafic and ultramafic rocks in Australia, Canada, Russia, and the US. Global cobalt mine and refinery production were forecast to increase to record high levels in 2022. The increase in raw materials feed was mainly from increased production at existing operations, although new production and restarts at suspended operations also contributed to supply. The DROC continued to be the world’s leading source of mined cobalt, supplying more than 70% of world cobalt mine production. China was the world’s leading producer of refined cobalt and a leading supplier of cobalt imports to the US. Much of China’s production comes from ore and partially refined cobalt imported from DROC; scrap and stocks of cobalt materials also contributed to China’s supply. China was the world’s leading consumer of cobalt, with more than 80% of its consumption being used by the rechargeable battery industry.
Substitutes: In some applications, substitution for cobalt would result in a loss in product performance. Potential substitutes include barium or strontium ferrites, neodymium-iron-boron, or nickel-iron alloys in magnets; cerium, iron, lead, manganese, or vanadium in paints; cobalt-iron-copper or iron-copper in diamond tools; copper-iron-manganese for curing unsaturated polyester resins; iron, iron-cobalt-nickel, nickel, cermets, or ceramics in cutting and wear resistant materials; iron-phosphorous, manganese, nickel-cobalt-aluminum, or nickel-cobalt-manganese in lithium-ion batteries; nickel-based alloys or ceramics in jet engines; nickel in petroleum catalysts; and rhodium in hydroformylation catalysts.
Note(s): More than 120 million tons of cobalt resources have been identified in manganese nodules and crusts on the floor of the Atlantic, Indian, and Pacific Oceans. Most US cobalt supply is comprised of imports and secondary (scrap) materials. In 2022, cobalt contained in purchased scrap represented an estimated 24% of estimated cobalt consumption.The mineral
cobaltite (cobalt sulfarsenide) (see attached image) is a valuable source of cobalt. The element is, however, more usually produced as a by-product of copper and nickel mining.
Copper (Cu/29)
Description: Copper is a mineral and an element. As a mineral, natural copper (also called native copper) is relatively rare. Copper is usually found in nature in association with sulfur. Chalcopyrite (see attached image) is a copper iron sulfide mineral and the most abundant copper ore mineral. Even though Chalcopyrite does not contain the most copper in its structure relative to other minerals, it is the most important copper ore since it can be found in many localities. Copper is one of the oldest metals ever used. Because of its properties of high ductility, malleability, conductivity, and resistance to corrosion, copper has become a major industrial metal, ranking third after iron and aluminum in terms of quantities consumed.
Uses: electric wire (motors, electromagnets, integrated circuits); plumbing (tubing, fittings); architectural roofing and features on buildings; alloys (brass, bronze)
US Imports: 825,000 mt (2022 est.) The US is 41% import reliant for its copper needs.
US Import Sources (2018-21): Copper content of blister and anodes: Finland, 90%; and other, 10%; Copper content of matte, ash, and precipitates: Canada, 34%; Belgium, 17%; Japan, 15%; Mexico, 11%. Copper content of ore and concentrates: Mexico, 82%; Canada, 18%; and other, <1%. Copper content of scrap: Canada,
51%; Mexico, 37%; and other, 12%. Refined copper: Chile, 64%; Canada, 20%; Mexico, 11%; and other, 5%. Refined copper accounted for 86% of all unmanufactured copper imports.
World Resources: A U.S. Geological Survey study of global copper deposits indicated that, as of 2015, identified resources contained 2.1 billion tons of copper, and undiscovered resources contained an estimated 3.5 billion tons. In 2022, the recoverable copper content of U.S. mine production was an estimated 1.3 million tons, an increase of 6% from that in 2021. Copper recovered from scrap contributed 32% of the U.S. copper supply.
Substitutes: Aluminum substitutes for copper in power cable, electrical equipment, automobile radiators, and cooling and refrigeration tubes. Titanium and steel are used in heat exchangers. Optical fiber substitutes for copper in telecommunications applications, and plastics substitute for copper in water pipe, drain pipe, and plumbing fixtures.
Gallium (Ga/31)
Description: Gallium is a metallic element that does not easily combine with other elements or ions to form ore minerals. It is, however, found as a trace element in a number of minerals and ores, the most important of which is bauxite, or aluminum ore (see attached image). In fact, gallium is a byproduct of alumina production. Gallium is not produced in the US, and demand is satisfied by imports. Imports of gallium metal and gallium arsenide (GaAs) wafers continued to account for all US consumption of gallium.
Uses: integrated circuits (cell phones, especially smart phones, wireless internet); optoelectronic devices (laser diodes, LEDs, photo-detectors, and solar cells); specialty alloys
US Imports: 562 mt (2022 est.). The US is 100% import reliant for its gallium needs.
US Import Sources (2018-21): metal - China, 53%; Germany and Japan, 13% each; Ukraine, 5%; and other, 16%.
World Resources: Globally, primary gallium is recovered as a byproduct of processing bauxite and zinc ores. Gallium contained in world resources of bauxite is estimated to exceed 1 million tons, and a considerable quantity could be contained in world zinc resources. However, less than 10% of the gallium in bauxite and zinc resources is potentially recoverable. In 2022, gallium metal imports increased by an estimated 34% from those in 2021 owing to increased imports from Canada, China, Slovakia, and the United Kingdom. Beginning in 2019, U.S. gallium metal imports decreased substantially from those in previous years because higher tariffs were placed on China’s gallium exports to the United States.
Substitutes: Liquid crystals made from organic compounds are used in visual displays as substitutes for LEDs. Silicon-based complementary metal-oxide semiconductor power amplifiers compete with GaAs power amplifiers in midtier 3G cellular handsets. Indium phosphide components can be substituted for GaAs-based infrared laser diodes in some specific-wavelength applications, and helium-neon lasers compete with GaAs in visible laser diode applications. Silicon is the principal competitor with GaAs in solar-cell applications. GaAs-based ICs are used in many defense-related applications because of their unique properties, and no effective substitutes exist for GaAs in these applications. GaAs in heterojunction bipolar transistors is being replaced in some applications by silicon-germanium.
Note(s): No US primary (low-grade, unrefined) gallium has been recovered since 1987.
Hafnium (Hf/72)
Description: Hafnium metal is produced when it is separated from zircon (see attached image), a zirconium silicate mineral that is usually 98% zirconium and 2% hafnium. Hafnium is a metallic element used in a number of industrial applications because of its resistance to corrosion and high temperatures.
Uses: control rods on nuclear reactors (primary use); nickel superalloys and high temperature alloys; integrated circuit production for features at 45mm and smaller; electrodes for plasma arc cutting
US Imports: 35 mt (2022 est.)
Import Sources (2018-21): Hafnium, unwrought: Germany, 36%; France, 30%; China, 26%; Russia, 3%; and other, 5%.
World Resources: World resources of hafnium are associated with those of zircon and baddeleyite. Quantitative estimates of hafnium resources are not available.
Substitutes: Silver-cadmium-indium control rods are used in lieu of hafnium at numerous nuclear power plants. Zirconium can be used interchangeably with hafnium in certain superalloys.
Note(s): Production of hafnium metal from hafnium oxide (HfO
2) at the Dubbo Zirconia Project, in New South Wales, Australia, would be independent of zirconium metal refinement for the nuclear industry where it is a byproduct. Additional heavy-mineral exploration and mining projects are underway in Australia, Mozambique, Sri Lanka, and Tanzania.
Indium (In/49)
Description: Indium is a soft, malleable, silvery-white metallic element; it is produced mainly from residues generated during zinc ore processing.
Uses: LCD displays; organic LEDs; fiber-optics; solder and alloys; infrared imaging; communications
US Imports: 160 mt (2022 est.). The US is 100% import reliant for its indium needs.
Import Sources (2018-21): Republic of Korea, 32%; Canada, 22%; China, 18%; France, 9%; and other, 19%.
World Resources: Indium is most commonly recovered from the zinc-sulfide ore mineral sphalerite (see attached image). The indium content of zinc deposits from which it is recovered ranges from less than 1 part per million to 100 parts per million.
Substitutes: Antimony tin oxide coatings have been developed as an alternative to indium tin oxide (ITO) coatings in LCDs and have been successfully annealed to LCD glass; carbon nanotube coatings have been developed as an alternative to ITO coatings in flexible displays, solar cells, and touch screens; PEDOT [poly(3,4-ethylene dioxythiophene)] has also been developed as a substitute for ITO in flexible displays and organic light-emitting diodes; and silver nanowires have been explored as a substitute for ITO in touch screens. Graphene has been developed to replace ITO electrodes in solar cells and also has been explored as a replacement for ITO in flexible touch screens. Researchers have developed a more adhesive zinc oxide nanopowder to replace ITO in LCDs. Gallium arsenide can substitute for indium phosphide in solar cells and in many semiconductor applications. Hafnium can replace indium in nuclear reactor control rod alloys.
Note(s): Indium was not recovered from ores in the US in 2022. Several companies produced indium products - including alloys, compounds, high-purity metal, and solders - from imported indium metal. Production of ITO continued to account for most of global indium consumption.
Lead (Pb/82)
Description: Lead is a very corrosion-resistant, dense, ductile, and malleable blue-gray metal that has been used for at least 5,000 years. The most significant lead mineral is galena (lead sulfide) (see attached image).
Uses: batteries; cable sheeting; solder; shielding (X-ray machines); ammunition
The lead-acid battery industry accounted for an estimated 92% of reported US lead consumption during 2021. Lead-acid batteries were primarily used as starting-lighting-ignition (SLI) batteries for automobiles, as industrial-type batteries for standby power for computer and telecommunications networks, and for motive power.
US Imports: 700,000 mt (2022 est.) The US is 42% import reliant for its lead needs.
Import Sources (2018-21): Refined metal: Canada, 42%; Mexico, 18%; Republic of Korea, 14%; and other, 26%.
World Resources: Identified world lead resources total more than 2 billion tons. In recent years, significant lead resources have been identified in association with zinc and (or) silver or copper deposits in Australia, China, Ireland, Mexico, Peru, Portugal, Russia, and the US (Alaska).
Substitutes: Substitution by plastics has reduced the use of lead in cable covering and cans. Tin has replaced lead in solder for potable water systems. The electronics industry has moved toward lead-free solders and flat-panel displays that do not require lead shielding. Steel and zinc are common substitutes for lead in wheel weights.
Note(s): According to the International Lead and Zinc Study Group, global refined lead production in 2022 was forecast to decrease by 0.3% to 12.34 million tons and refined lead consumption to increase by 0.8% to 12.42 million tons.
Lithium (Li/3)
Description: Lithium is a metallic element widely distributed in the earth's crust at low concentrations. Spodumene (see attached image), petalite (see attached image), and lepidolite (see attached image) are important mineral sources of lithium. Subsurface brines are the dominant raw material for lithium carbonate production worldwide because of lower production costs as compared with the costs for hard rock ores.
Uses: enamels; glass; ceramics; air purification; lithium ion-batteries; focal lenses for telescopes
Although lithium markets vary by location, global end-use markets are estimated as follows: batteries, 74%; ceramics and glass, 14%; lubricating greases, 3%; continuous casting mold flux powders, 2%; polymer production, 2%; air treatment, 1%; and other uses, 4%. Lithium consumption for batteries has increased significantly in recent years because rechargeable lithium batteries are used extensively in the growing market for electric vehicles and portable electronic devices, and increasingly are used in electric tools, and grid storage applications. Lithium minerals were used directly as ore concentrates in ceramics and glass applications.
US Imports: 3,400 mt (2022 est.) The US is more than 25% import reliant for its lithium needs.
Import Sources (2018-21): Argentina, 51%; Chile, 40%; China, 4%; Russia, 3%; and other, 2%.
World Resources: Owing to continuing exploration, identified lithium resources have increased substantially worldwide and total about 98 million tons. Identified lithium resources in the United States—from continental brines, claystone, geothermal brines, hectorite, oilfield brines, and pegmatites—are 12 million tons. Identified lithium resources in other countries have been revised to 86 million tons. Identified lithium resources are distributed as follows: Bolivia, 21 million tons; Argentina, 20 million tons; Chile, 11 million tons; Australia, 7.9 million tons; China, 6.8 million tons; Germany, 3.2 million tons; DROC, 3 million tons; Canada, 2.9 million tons; Mexico, 1.7 million tons; Czechia, 1.3 million tons; Serbia, 1.2 million tons; Russia, 1 million tons; Peru, 880,000 tons; Mali, 840,000 tons; Brazil, 730,000 tons; Zimbabwe, 690,000 tons; Spain, 320,000 tons; Portugal, 270,000 tons; Namibia, 230,000 tons; Ghana, 180,000 tons; Finland, 68,000 tons; Austria, 60,000 tons; and Kazakhstan, 50,000 tons.
Substitutes: Substitution for lithium compounds is possible in batteries, ceramics, greases, and manufactured glass. Examples are calcium, magnesium, mercury, and zinc as anode material in primary batteries; calcium and aluminum soaps as substitutes for stearates in greases; and sodic and potassic fluxes in ceramics and glass manufacture.
Note(s): Lithium supply security has become a top priority for technology companies in the US and Asia. Strategic alliances and joint ventures among technology companies and exploration companies continue to be established to ensure a reliable, diversified supply of lithium for battery suppliers and vehicle manufacturers.
Magnesium (Mg/12)
Description: Magnesium is the eighth most abundant element in the Earth’s crust, representing about 2% of its total mass; it belongs to the alkaline earth metal series. The metal is silvery white and is prized for its lightness and strength in alloys. Magnesium can be extracted from the minerals dolomite (see attached Image) and carnallite, but it is most often obtained from seawater or well and lake brines.
Uses: refractory material in furnace linings for steel, iron, metals, glass and cement production; used in agricultural, chemical, and construction industries
The leading use for primary magnesium metal, which accounted for 58% of reported domestic US consumption, was in castings, principally used for the automotive industry.
US Imports: 97,000 mt (2022 est.) The US is more than 50% import reliant for its magnesium metal needs.
Import Sources (2018-21): Canada, 21%; Israel, 11%; Mexico, 10%; Taiwan, 9%; and other, 49%.
World Resources: Resources from which magnesium may be recovered range from large to virtually unlimited and are globally widespread. Resources of dolomite, serpentine, and magnesium-bearing evaporite minerals are enormous. Magnesium-bearing brines are estimated to constitute a resource in the billions of tons, and magnesium could be recovered from seawater along world coastlines.
Substitutes: Aluminum and zinc may substitute for magnesium in castings and wrought products. The relatively light weight of magnesium is an advantage over aluminum and zinc in castings and wrought products in most applications; however, its high cost is a disadvantage relative to these substitutes. For iron and steel desulfurization, calcium carbide may be used instead of magnesium. Magnesium is preferred to calcium carbide for desulfurization of iron and steel because calcium carbide produces acetylene in the presence of water.
Note(s): The use of magnesium in automobile parts continued to increase as automobile manufacturers sought to decrease vehicle weight for increased fuel efficiency.
Manganese (Mn/25)
Description: Manganese is a very brittle but hard metallic element. Pyrolusite (manganese dioxide (MnO2) (see attached image) is the main ore mineral for manganese. Rhodochrosite (see attached image) is also used as an ore of manganese but is rarely found in economic quantities. Manganese is essential to iron and steel production by virtue of its sulfur-fixing, deoxidizing, and alloying properties.
Uses: steelmaking; aluminum alloy production; additive in unleaded gasoline; glass making and coloring; batteries and dry cells; alloys for chemical processing applications; alloys for high temperature bolts and fasteners
US Imports: Manganese ore 650,000 mt (2022 est.). The US is 100% import reliant for its manganese needs.
Import Sources (2018-21): Manganese ore: Gabon, 67%; South Africa, 19%; Mexico, 12%; and other, 2%.
World Resources: Land-based manganese resources are large but irregularly distributed. South Africa accounts for about 30% of the world’s manganese reserves. Those in the US are very low grade and have potentially high extraction costs. Global production of manganese ore was estimated to be unchanged from that in 2021. The leading countries for manganese ore production were, in descending order on a contained-weight basis, South Africa, Gabon, and Australia.
Substitutes: Manganese has no satisfactory substitute in its major applications.
Mercury (Hg/80)
Description: Mercury is the only common metal that is liquid at room temperature. It occurs either as native metal or in cinnabar (see attached image), corderoite, livingstonite, and other minerals. Mercury has uniform volumetric thermal expansion, good electrical conductivity, and easily forms amalgams with almost all common metals except iron.
Uses: munition fuzes; missile and space guidance system gyroscopes; dental equipment; electric lighting; infrared detection
US Imports: 2 mt (2022)
Import Sources (2018-21): Canada, 69%; China, 31%; and other, <1%.
World Resources: China, Kyrgyzstan, Mexico, Peru, Russia, Slovenia, Spain, and Ukraine have most of the world’s estimated 600,000 tons of mercury resources. Mexico reclaims mercury from Spanish colonial silver-mining waste. In Spain, once a leading producer of mercury, mining at its centuries-old Almaden Mine stopped in 2003. In the US, there are mercury occurrences in Alaska, Arkansas, California, Nevada, and Texas. The declining consumption of mercury, except for small-scale gold mining, indicates that these resources are sufficient for centuries of use.
Substitutes: Ceramic composites substitute for the dark-gray mercury-containing dental amalgam. “Galistan,” an alloy of gallium, indium, and tin, replaces the mercury used in traditional mercury thermometers, and digital thermometers have replaced traditional thermometers. At chloralkali plants around the world, mercury-cell technology is being replaced by newer diaphragm and membrane cell technology. LEDs that contain indium substitute for mercury-containing fluorescent lamps. Lithium, nickel-cadmium, and zinc-air batteries replace mercury-zinc batteries in the US; indium compounds substitute for mercury in alkaline batteries; and organic compounds have been substituted for mercury fungicides in latex paint.
Note(s): Owing to mercury toxicity and concerns for the environment and human health, overall mercury use has declined in the US. Mercury continues to be released to the environment from numerous sources, including mercury-containing car switches when automobiles are scrapped without recovering them for recycling, coal-fired power plant emissions, and incineration of mercury-containing medical devices. Mercury is no longer used in batteries and paints manufactured in the US.
Molybdenum (Mo/42)
Description: Molybdenum is a refractory metallic element used principally as an alloying agent in steel, cast iron, and superalloys to enhance hardenability, strength, toughness, and wear and corrosion resistance. The mineral molybdenite (molybdenum sulfide) (see attached image) is an important source of molybdenum.
Uses: component in tool and alloy steels; component in nickel superalloys for high temperature sections of jet engines; lubricant; colorant; nickel superalloys for high temperature sections of turbine engines
US Imports: 33,000 mt (2022 est.)
Import Sources (2018-21): Molybdenum ores and concentrates: Peru, 60%; Mexico, 15%; Chile, 14%; and other, 11%.
World Resources: Global molybdenum production in 2022 was essentially unchanged compared with that in 2021. In descending order of production, China, Chile, the United States, Peru, and Mexico provided 93% of total global production. Identified resources of molybdenum in the US are about 5.4 million tons, and in the rest of the world, about 20 million tons. Molybdenum occurs as the principal metal sulfide in large low-grade porphyry molybdenum deposits and as an associated metal sulfide in low-grade porphyry copper deposits. Resources of molybdenum are adequate to supply world needs for the foreseeable future.
Substitutes: There is little substitution for molybdenum in its major application in steels and cast irons. Potential substitutes include boron, chromium, niobium (columbium), and vanadium in alloy steels; tungsten in tool steels; graphite, tantalum, and tungsten for refractory materials in high-temperature electric furnaces; and cadmium-red, chrome-orange, and organic-orange pigments for molybdenum orange.
Nickel (Ni/28)
Description: Nickel is a silvery metallic element. Most of the nickel mined comes from two types of deposits: laterites where the principal minerals are nickeliferous limonite (hydrated iron oxide) (see attached image) and garnierite (hydrous nickel silicate) (see attached image), or magmatic sulfide deposits where the principal mineral is pentlandite (iron nickel sulfide) (see attached image).
Uses: land based turbines; turbines for jet aircraft engines; turbines for large-scale power generation; liquid gas storage; high speed steels
US Imports: 164,000 mt (2022 est.) The US is 56% import reliant for its nickel needs.
Import Sources (2018-21): Primary nickel: Canada, 45%; Norway, 9%; Australia, 8%; Finland, 7%; and other, 31%. Nickel-containing scrap, including nickel content of stainless-steel scrap: Canada, 38%; Mexico, 26%; United Kingdom, 9%; and other, 27%.
World Resources: Identified land-based resources averaging 1% nickel or greater contain at least 130 million tons of nickel, with about 60% in laterites and 40% in sulfide deposits. Extensive nickel resources also are found in manganese crusts and nodules on the ocean floor. The decline in discovery of new sulfide deposits in traditional mining districts has led to exploration in more challenging locations such as east-central Africa and the subarctic. In 2022, estimated global nickel mine production increased by about 20%, with almost all of the increased production attributed to Indonesia.
Substitutes: Low-nickel, duplex, or ultrahigh-chromium stainless steels are being substituted for austenitic grades in construction. Nickel-free specialty steels are sometimes used in place of stainless steel in the power-generating and petrochemical industries. Titanium alloys can substitute for nickel metal or nickel-base alloys in corrosive chemical environments. Lithium-ion batteries may be used instead of nickel metal hydride batteries in certain applications.
Note(s): In 2022, the annual average London Metal Exchange nickel cash price was estimated to have increased by 35% compared with that in 2021 which was attributed to increasing use of nickel in electric vehicle batteries and continued strong demand for stainless steel. In the United States, the leading uses for primary nickel are alloys and steels, electroplating, and other uses including catalysts and chemicals. Stainless and alloy steel and nickel-containing alloys typically account for more than 85% of domestic consumption.
Niobium (Nb/41)
Description: Columbium and niobium are synonymous names for the chemical element with atomic number 41; columbium was the name given in 1801, and niobium (Nb) was the name officially designated by the International Union of Pure and Applied Chemistry in 1950. The US does not have a niobium mining industry because identified resources are low grade. Brazil and Canada are the major producers of niobium mineral concentrates. Niobium is often found in the minerals pyrochlore (see attached image) and columbite (see attached image).
Uses: alloying element in steels, stainless steels, superalloys (nickel, cobalt, and iron-based); jet engine components; gas turbines; heat resistant and combustion equipment; tool bits and cutting tools
US Imports: 8,800 mt (2022 est.). The US is 100% import reliant for its niobium needs.
US Import Sources (2018-21): Niobium and tantalum ores and concentrates: Australia, 42%; Rwanda, 21%; DROC, 12%; Mozambique, 7%; and other, 18%. Niobium oxide: Brazil, 72%; Estonia, 5%; China, 2%; Germany, 1%; and other, 20%. Ferroniobium and niobium metal: Brazil, 67%; Canada, 28%; Russia, 3%, Germany, 1%, and other, 1%. Total imports: Brazil, 66%; Canada, 25%; and other, 9%. Of U.S. niobium material imports (by contained weight), 74% was ferroniobium, 16% was niobium metal, 9% was niobium oxide, and 1% was niobium ores and concentrates.
World Resources: Brazil continued to be the world's leading niobium producer, accounting for approximately 89% of global production, followed by Canada with about 8%. World resources of niobium are more than adequate to supply projected needs. Most of the world’s identified resources of niobium occur as pyrochlore in carbonatite (igneous rocks that contain more than 50%-by-volume carbonate minerals) deposits and are outside the United States.
Substitutes: The following materials can be substituted for niobium, but a performance loss or higher cost may ensue: molybdenum and vanadium, as alloying elements in high-strength low-alloy steels; tantalum and titanium, as alloying elements in stainless- and high-strength steels; and ceramics, molybdenum, tantalum, and tungsten in high temperature applications.
Note(s): Significant US niobium mine production has not been reported since 1959. Niobium principally is imported in the form of ferroniobium.
Rhenium (Re/75)
Description: Rhenium is a silvery-gray, heavy, transition metal. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the earth's crust. Rhenium has the third-highest melting point and second-highest boiling point of any element. Rhenium resembles manganese and technetium chemically and is mainly obtained as a by-product of the extraction and refinement of molybdenum and copper ores.
Uses: superalloys used in high-temperature turbine engine components; petroleum-reforming catalysts
US Imports: 10 mt (2022 est.) The US is 69% import reliant for its rhenium needs.
Import Sources (2018-21): Ammonium perrhenate: Kazakhstan, 27%; Poland, 16%; Canada, 15%; Germany, 15%; and other, 27%. Rhenium metal powder: Chile, 84%; Canada, 8%; Germany, 6%; and other, 2%. Total imports: Chile, 63%; Canada, 10%; Germany, 8%; Kazakhstan, 7%; and other, 12%.
World Resources: Most rhenium occurs with molybdenum in porphyry copper deposits. Identified US resources are estimated to be about 5 million kilograms, and the identified resources of the rest of the world are approximately 6 million kilograms. Rhenium also is associated with copper minerals in sedimentary deposits in Armenia, Kazakhstan, Poland, Russia, and Uzbekistan, where ore is processed for copper recovery and the rhenium-bearing residues are recovered at copper smelters. World rhenium production in 2022 decreased slightly compared with that in 2021.
Substitutes: Substitutes for rhenium in platinum-rhenium catalysts are being evaluated continually. Iridium and tin have achieved commercial success in one such application. Other metals being evaluated for catalytic use include gallium, germanium, indium, selenium, silicon, tungsten, and vanadium. The use of these and other metals in bimetallic catalysts might decrease rhenium’s share of the existing catalyst market; however, this would likely be offset by rhenium-bearing catalysts being considered for use in several proposed gas-to-liquid projects. Materials that can substitute for rhenium in various end uses are as follows: cobalt and tungsten for coatings on copper x-ray targets, rhodium and rhodium-iridium for high-temperature thermocouples, tungsten and platinum-ruthenium for coatings on electrical contacts, and tungsten and tantalum for electron emitters.
Note(s): In 2022, apparent consumption in the United States was 8% less than that in 2021. During 2022, the United States continued to rely on imports for much of its supply of rhenium. Canada, Chile, Germany, Kazakhstan, and Poland supplied most of the imported rhenium.
Rubidium (Rb/37)
Description: Rubidium is a soft, ductile, silvery-white metal that melts at 39.3 °C. Naturally occurring rubidium is slightly radioactive. Rubidium is an extremely reactive metal - it ignites spontaneously in the presence of air and decomposes water explosively, igniting the liberated hydrogen. Lepidolite (see attached image) contains between 0.3% and 3.5% rubidium, and is the commercial source of the element.
Uses: Rubidium is used interchangeably or together with cesium in many uses; its principal application is in specialty glasses used in fiber optic telecommunication systems; Rubidium’s photoemissive properties have led to its use in night-vision devices, photoelectric cells, and photomultiplier tubes; it has several uses in medical science, such as in positron emission tomographic (PET) imaging, the treatment of epilepsy, and the ultracentrifugal separation of nucleic acids and viruses.
US imports: US salient statistics, such as consumption, exports, and imports, are not available. Some concentrate was imported to the US for further processing. Industry information during the past decade suggests a domestic consumption rate of approximately 2,000 kilograms per year. The US was 100% import reliant for rubidium minerals.
Import sources: No reliable data has been available to determine the source of rubidium ore imported by the United States since 1988. Prior to 2016, Canada was thought to be the primary supplier of rubidium ore.
World Resources: Although rubidium is more abundant in the earth’s crust than copper, lead, or zinc, it forms no minerals of its own, and is, or has been, produced in small quantities as a byproduct of the processing of cesium and lithium ores taken from a few small deposits in Canada, Namibia, and Zambia.
Substitutes: Cesium and rubidium can be used interchangeably in many applications because they have similar physical properties and atomic radii.
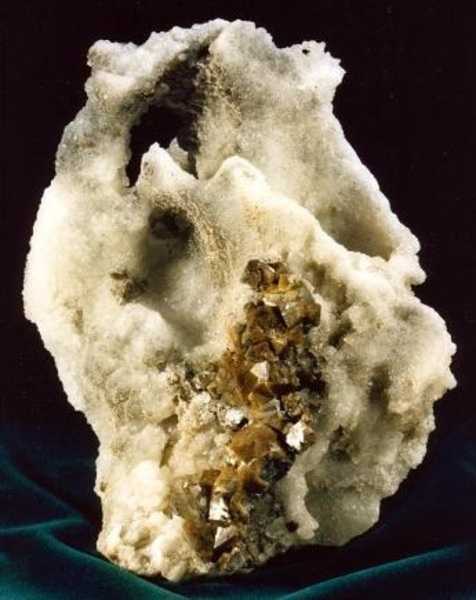
Strontium (Sr/38)
Description: Strontium is a silvery-white metal, found in nature in two minerals, celestite (strontium sulfate) (see attached image) and strontianite (strontium carbonate) (see attached image).
Uses: alloys; pyrotechnics; ceramics and glasses; electrolytic production of zinc; tracer ammunition
US Imports: strontium compounds – 5,100 mt; Celestite - 7,200 mt (2022 est.). The US is 100% import reliant for its strontium needs.
Import Sources (2018-21): Celestite: Mexico, 100%. Strontium compounds: Germany, 47%; Mexico, 45%; China, 4%; and other, 4%. Total imports: Mexico, 75%; Germany, 21%; China, 2%; and other, 2%.
World Resources: World resources of strontium are thought to exceed 1 billion tons. All of the celestite is imported from Mexico and is thought to be used exclusively as an additive in drilling fluids for oil and natural gas exploration and production. For these applications, celestite is ground but undergoes no chemical processing. Outside the US, celestite is the raw material used for production of strontium compounds.
Substitutes: Barium can be substituted for strontium in ferrite ceramic magnets; however, the resulting barium composite will have reduced maximum operating temperature when compared with that of strontium composites. Substituting for strontium in pyrotechnics is hindered by difficulty in obtaining the desired brilliance and visibility imparted by strontium and its compounds.
Note(s): Apparent consumption of total strontium increased significantly in 2022. Apparent consumption of strontium compounds increased slightly, but apparent consumption of celestite increased to within 10% of that in 2019. The increase in 2022 was likely the result of improved economic conditions following the economic downturn attributed to the global coronavirus disease 2019 (COVID-19) pandemic in 2020, and the prolonged economic recovery period in 2021. World celestite production in 2022 was estimated to have remained essentially unchanged from that in 2021. Strontium carbonate is sintered with iron oxide to produce permanent ceramic ferrite magnets. Strontium nitrate contributes a brilliant red color to fireworks and signal flares.
Tantalum (Ta/73)
Description: Tantalum is a metallic element that is ductile, easily fabricated, highly resistant to corrosion by acids, and a good conductor of heat and electricity with a high melting point. Tantalum occurs in geologic sources together with the chemically similar niobium, mainly in the mineral groups tantalite (see attached image) and columbite (see attached image). The major use for tantalum, as tantalum metal powder, is in the production of electronic components, mainly tantalum capacitors. Major end uses for tantalum capacitors include portable telephones, pagers, personal computers, and automotive electronics. Alloyed with other metals, tantalum is also used in making carbide tools for metalworking equipment and in the production of superalloys for jet engine components.
Uses: chemical processing equipment; heat exchangers; anti-lock brake systems; high temperature aerospace engine parts; night vision goggles; global positioning systems; missile systems
US Imports: 1,700 mt (2022 est.). The US is 100% import reliant for its tantalum needs.
Import Sources (2018-21): Tantalum ores and concentrates: Australia, 43%; Rwanda, 21%; Congo (Kinshasa), 12%; Mozambique, 7%; and other, 17%. Tantalum metal and powder: China,6 42%; Germany, 23%; Kazakhstan, 12%; Thailand, 9%; and other, 14%. Tantalum waste and scrap: Indonesia, 23%; China,6 17%; Japan, 15%; and other, 45%. Total: China,6 24%; Germany, 12%; Australia, 10%; Indonesia, 8%; and other, 46%.
World Resources: Identified resources of tantalum, most of which are in Australia, Brazil, and Canada, are considered adequate to meet projected needs. Brazil, DROC, Nigeria, and Rwanda accounted for about 85% of estimated global tantalum production in 2022. The US has about 55,000 tons of tantalum resources in identified deposits, most of which are considered uneconomic at 2022 prices.
Substitutes: The following materials can be substituted for tantalum, but usually with less effectiveness: niobium in carbides; aluminum and ceramics in electronic capacitors; glass, niobium, platinum, titanium, and zirconium in corrosion-resistant applications; and hafnium, iridium, molybdenum, niobium, rhenium, and tungsten in high-temperature applications.
Note(s): No significant US tantalum mine production has been reported since 1959. Domestic tantalum resources are of low grade, some are mineralogically complex, and most are not commercially recoverable. Companies in the US produce tantalum alloys, capacitors, compounds, and metal from imported tantalum ores and concentrates, tantalum-containing materials, and metal and alloys recovered from foreign and domestic scrap.
Tin (Sn/50)
Description: Tin is a silvery-white metallic element. The most important ore mineral of tin, cassiterite (tin dioxide) (see attached image), is formed in high-temperature veins that are usually related to igneous rocks such as granites and rhyolites. It is often found in association with tungsten minerals.
Uses: bearings; containers; solder; bronze; chemicals; LCD TVs, touch screens and portable electronics
US Imports: refined – 34,000 mt (2022 est.) The US is 77% import reliant for its tin needs.
Import Sources (2018-21): Refined tin: Peru, 25%; Indonesia, 24%; Bolivia, 17%; Malaysia, 16%; and other, 18%.
World Resources: World resources, principally in western Africa, southeastern Asia, Australia, Bolivia, Brazil, Indonesia, and Russia, are extensive and, if developed, could sustain recent annual production rates well into the future.
Substitutes: Aluminum, glass, paper, plastic, or tin-free steel substitute for tin content in cans and containers. Other materials that substitute for tin are epoxy resins for solder; aluminum alloys, alternative copper-base alloys, and plastics for bronze; plastics for bearing metals that contain tin; and compounds of lead and sodium for some tin chemicals.
Note(s): Identified resources of tin in the US, primarily in Alaska, are insignificant compared with those of the rest of the world. Tin has not been mined or smelted in the US since 1993 and 1989, respectively.
Titanium (Ti/22)
Description: Titanium is a hard, silvery-white metallic element. As a metal (see attached image), titanium is well known for corrosion resistance and for its high strength-to-weight ratio. When titanium metal is produced from ore, it is first produced in sponge form before it is melted into metal shapes. Titanium dioxide pigment is a white pigment characterized by its purity, refractive index, particle size, and surface properties. Titanium metal and pigment are produced from the minerals ilmenite (see attached image), leucoxene, and rutile (see attached image).
Uses: landing gear, springs, rotors (helicopter), fittings, and attachments; structural components for airplanes, satellites, and spacecraft; gas turbine engines; chemical processing
US Imports: Sponge metal: 28,000 mt, Titanium dioxide pigment: 260,000 mt (2022 est.) The US is 81% import reliant for its titanium mineral concentrate needs, and more than 95% import reliant for titanium sponge.
Import Sources (2018-21): Sponge metal: Japan, 89%; Kazakhstan, 9%; Ukraine, 1%, and other, 1%. Titanium dioxide pigment: Canada, 42%; China, 16%; Germany, 10%; Belgium, 5%; and other, 27%.
World Resources: Ilmenite accounts for about 90% of the world’s consumption of titanium minerals. In 2022, China continued to be the leading producer and consumer of titanium mineral concentrates, accounting for more than one-third of global production of ilmenite. Mozambique and South Africa also were leading producers of titanium mineral concentrates. World resources of anatase (see attached image), ilmenite, and rutile total more than 2 billion tons.
Substitutes: Few materials possess titanium metal’s strength-to-weight ratio and corrosion resistance. In high-strength applications, titanium competes with aluminum, composites, intermetallics, steel, and superalloys. Aluminum, nickel, specialty steels, and zirconium alloys may be substituted for titanium for applications that require corrosion resistance. Ground calcium carbonate, precipitated calcium carbonate, kaolin, and talc compete with titanium dioxide as a white pigment.
Note(s): Rebounding demand from the aerospace and other industries resulted in a 75% increase in imports of titanium sponge compared with those in 2021. Japan (82%), Kazakhstan (9%), and Saudi Arabia (7%) were the leading import sources for titanium sponge in 2022.
Tungsten (W/74)
Description: Tungsten is a gray-white metallic element; it has the highest melting temperature of all elements except carbon and is one of the heaviest elements. Tungsten is produced from the mineral ores scheelite (calcium tungstate) (see attached image) and wolframite (iron-manganese tungstate) (see attached image). The ore is concentrated and then usually produced into the intermediate product ammonium paratungstate (APT) before being processed into metallic applications. The US does not have any operating tungsten mines.
Uses: steels; wear-resistant alloys; component in nickel superalloys for high-temperature sections of jet engines; armor penetrating projectiles; aircraft weights and counterweights; small arms ammunition
US Imports: 14,000 mt (2022 est.) The US is more than 50% import reliant for its tungsten needs.
Import Sources (2018-21): Tungsten contained in ores and concentrates, intermediate and primary products, wrought and unwrought tungsten, and waste and scrap: China, 29%; Germany, 11%; Bolivia, 9%; Vietnam, 6%; and other, 45%.
World Resources: World tungsten resources are geographically widespread. China ranks first in the world in terms of tungsten resources and reserves and has some of the largest deposits. Canada, Kazakhstan, Russia, and the US also have significant tungsten resources.
Substitutes: Potential substitutes for cemented tungsten carbides include cemented carbides based on molybdenum carbide and titanium carbide, ceramics, ceramic-metallic composites (cermets), and tool steels. Potential substitutes for other applications are as follows: molybdenum for certain tungsten mill products; molybdenum steels for tungsten steels; lighting based on carbon nanotube filaments, induction technology, and light-emitting diodes for lighting based on tungsten electrodes or filaments; depleted uranium or lead for tungsten or tungsten alloys in applications requiring high-density or the ability to shield radiation; and depleted uranium alloys or hardened steel for cemented tungsten carbides or tungsten alloys in armor-piercing projectiles.
Note(s): World tungsten supply was dominated by production in China and exports from China. Production of tungsten concentrate outside China was estimated to have increased in 2022 but remained less than 20% of world production. The increase was from existing operations. Additional production, primarily from reopened mines in Australia, the Republic of Korea, and the United Kingdom, was forecast to begin in 2023. Scrap continued to be an important source of raw material for the world tungsten industry. China continued to be the world’s leading tungsten consumer. There has been no known US domestic commercial production of tungsten concentrates since 2015.
Uranium (U/92)
Description: Uranium is a common metal found in rocks all over the world. Uranium (see attached image) occurs in combination with small amounts of other elements. Uranium ranks 48th among the most abundant elements found in natural crustal rocks. It is 1.67 times more dense than lead.
Uses: Uranium is the fuel most widely used by nuclear power plants for nuclear fission. In nuclear fission, energy is released when atoms are split apart to form smaller atoms. Nuclear power plants use the heat from nuclear fission to produce electricity.
US Imports: 20,077 mt (2021)
Import Sources (2021): Canada, 15.6%; Kazakhstan, 37.4%; Russia, 14.3%; Australia, 15%; Namibia, 7.3%; other, 10.3%
World Resources: Economically recoverable uranium deposits have been discovered primarily in the western US, Australia, Canada, Central Asia, Africa, and South America. About 5.3% of the uranium delivered to US reactors in 2021 was produced in the US and 94.7% came from other countries.
Substitutes: None
Note(s): Nuclear power plants use a certain type of uranium, U-235, as fuel because its atoms are easily split apart. Although uranium is about 100 times more common than silver, U-235 is relatively rare. After uranium is mined, the U-235 must be extracted and processed before it can be used as a fuel. Mined uranium ore typically yields 0.5 to 2 kg (1 to 4 pounds) of uranium oxide concentrate (U3O8 or yellowcake) per ton, or 0.05% to 0.20% yellowcake.
Vanadium (V/23)
Description: Vanadium is a soft, silver-gray metallic element. There is no single mineral ore from which vanadium is recovered. However, it is found as a trace element in a several types of rock and is a by-product of other mining operations. Vanadinite (lead chlorovanadate) (see attached image) is mineral that contains vanadium.
Uses: steel; Titanium-Aluminum-Vanadium alloys in jet engines and high-speed aircraft; cladding titanium to steel; energy storage
US Imports: Aluminum-vanadium master alloy 30 mt; Ferrovanadium 2,700 mt; Vanadium pentoxide 1,500 mt; Vanadium ores and concentrates 67 mt (2022 est.) The US is 54% import reliant for its vanadium needs.
Import Sources (2018-21): Ferrovanadium: Austria, 38%; Canada, 38%; Russia, 10%; Japan, 4%; and other, 10%. Vanadium pentoxide: Brazil, 50%; South Africa, 32%; China, 6%; Russia, 4%; and other, 8%. Total: Canada, 31%; China, 13%; Brazil, 8%; South Africa, 7%; and other, 41%.
World Resources: World resources of vanadium exceed 63 million tons. Vanadium occurs in deposits of phosphate rock, titaniferous magnetite, and uraniferous sandstone and siltstone, in which it constitutes less than 2% of the host rock. Significant quantities are also present in bauxite and carboniferous materials, such as coal, crude oil, oil shale, and tar sands. Because vanadium is typically recovered as a byproduct or coproduct, demonstrated world resources of the element are not fully indicative of available supplies.
Substitutes: Steels containing various combinations of other alloying elements can be substituted for steels containing vanadium. Certain metals, such as manganese, molybdenum, niobium (columbium), titanium, and tungsten, are to some degree interchangeable with vanadium as alloying elements in steel. Platinum and nickel can replace vanadium compounds as catalysts in some chemical processes. Currently, no acceptable substitute for vanadium is available for use in aerospace titanium alloys.
Note(s): Estimated US apparent consumption of vanadium in 2022 increased by 11% from that in 2021. Metallurgical use, primarily as an alloying agent for iron and steel, accounted for about 94% of US domestic vanadium consumption in 2022.
Zinc (Zn/30)
Description: Zinc is a blue-gray, metallic element; it is recovered from a number of different zinc minerals, the most significant of which is sphalerite (zinc sulfide) (see attached image). Other minerals, such as cassiterite (see attached image), smithsonite (zinc carbonate) and zincite (zinc oxide), are also zinc ores.
Uses: galvanized steel; bronze and brass; solder; batteries; solar cells
US Imports: Ore and refined metal – 706,000 mt (2022 est.) The US is 77% import reliant for its zinc needs.
Import Sources (2018-21): Ores and concentrates: Peru, 71%; Canada, 15%; China, 7%, Taiwan, 4%; and other, 3%. Refined metal: Canada, 66%; Mexico, 16%; Peru, 6%; Spain, 6%; and other, 6%.
World Resources: Identified zinc resources of the world are about 1.9 billion tons. According to the International Lead and Zinc Study Group, estimated global refined zinc production in 2022 was forecast to decrease by 2.7% to 13.49 million tons and estimated metal consumption to decrease by 1.9% to 13.79 million tons, resulting in a production-to-consumption deficit of 297,000 tons. US zinc mine production increased by 9% in 2022 compared with that in 2021.
Substitutes: Aluminum and plastics substitute for galvanized sheet in automobiles; aluminum alloys, cadmium, paint, and plastic coatings replace zinc coatings in other applications. Aluminum- and magnesium-base alloys are major competitors for zinc-base die-casting alloys. Many elements are substitutes for zinc in chemical, electronic, and pigment uses.
Zirconium (Zr/40)
Description: Zircon (see attached image) is a zirconium silicate mineral that is usually 98% zirconium and 2% hafnium and is the primary source of both materials. Zirconium is a metallic element used in a number of industrial applications because it is so resistant to corrosion and high temperatures.
Uses: space vehicles and parts; abrasives; alloys for naval applications; metallurgical furnaces; ceramic knives; artificial joints and limbs
US Imports: Zirconium ores and concentrates 35,000 mt; Zirconium, unwrought 290 mt; Zirconium, wrought 300 mt (2022 est.) The US is less than 50% import reliant for its zirconium needs.
Import Sources (2018-21): Zirconium ores and concentrates: South Africa, 51%; Senegal, 25%; Australia, 21%; Russia, 2%; and other, 1%. Zirconium, unwrought: China, 89%; Germany, 8%; France, 1%; Japan, 1%, and other, 1%. Zirconium, wrought: France, 62%; Germany, 19%; Belgium, 6%; Canada, 4%; and other, 9%.
World Resources: Resources of zircon in the US included about 14 million tons associated with titanium resources in heavy-mineral-sand deposits. Phosphate rock and sand and gravel deposits could potentially yield substantial amounts of zircon as a byproduct.
Substitutes: Chromite and olivine can be used instead of zircon for some foundry applications. Dolomite and spinel refractories can also substitute for zircon in certain high-temperature applications.
Note(s): The leading consumers of zirconium metal are the chemical process and nuclear energy industries. US imports and exports of zirconium ores and concentrates increased significantly in 2022. Australia, Senegal, and South Africa continued to be the leading import sources of zirconium ores and mineral concentrates.
Non-metals
Arsenic (As/33)
Description: Arsenic is a gray, yellow, or black metalloid that is generally recovered as a by-product from other metal processing. The brittle gray form used by industry is the most common form.
Uses: high-purity arsenic (99.9999%) is used to produce Gallium-Arsenide (GaAs) semiconductors for solar cells, space research, and telecommunications; used for Germanium-Arsenide-Selenide specialty optical materials; Indium-Gallium-Arsenide (InGaAs) is used for short-wave infrared technology; lead-hardening alloy for use in ammunition and batteries; pesticides, herbicides, Chromated-Copper-Arsenide (CCA) wood preservative
US imports: Arsenic metal 790 mt, Arsenic compounds 4,600 mt (2022 est.). The US is 100% import reliant for its arsenic needs.
Import Sources (2018-21): all forms of Arsenic: China, 57%; Morocco, 35%; Belgium, 4%; and other, 4%.
World Resources: Arsenic may be obtained from copper, gold, and lead smelter flue dust, as well as from roasting arsenopyrite, the most abundant ore mineral of arsenic. Arsenic has been recovered from orpiment and realgar in China, Peru, and the Philippines, as well as from copper-gold ores in Chile; it was associated with gold occurrences in Canada. Orpiment and realgar from gold mines in Sichuan Province, China, were stockpiled for later recovery of arsenic. Arsenic also may be recovered from enargite, a copper mineral. Arsenic trioxide was produced at the hydrometallurgical complex of Guemassa, near Marrakech, Morocco, from cobalt arsenide ore from the Bou-Azzer Mine.
Substitutes: Substitutes for CCA in wood treatment include alkaline copper quaternary, ammoniacal copper quaternary, ammoniacal copper zinc arsenate, alkaline copper quaternary boron-based preservatives, copper azole, copper citrate, and copper naphthenate. Treated wood substitutes include concrete, plastic composite material, plasticized wood scrap, or steel. Silicon-based complementary metal-oxide semiconductor power amplifiers compete with GaAs power amplifiers in mid-tier 3G cellular handsets. Indium phosphide components can be substituted for GaAs-based infrared laser diodes in some specific-wavelength applications, and helium-neon lasers compete with GaAs in visible laser diode applications. Silicon is the principal competitor with GaAs in solar-cell applications. GaAs-based integrated circuits are used in many defense-related applications because of their unique properties, and no effective substitutes exist for GaAs in these applications. GaAs in heterojunction bipolar transistors is being replaced in some applications by silicon-germanium.
Note(s): Arsenic trioxide and primary arsenic metal have not been produced in the United States since 1985. Peru, China, and Morocco, in descending order, continued to be the leading global producers of arsenic trioxide, accounting for about 93% of estimated world production in 2022. China and Morocco continued to supply about 93% of US imports of arsenic trioxide in 2022. China was the leading world producer of arsenic metal and supplied about 97% of US arsenic metal imports in 2022.
Boron (B/5)
Description: Boron is a relatively rare element representing only 0.001% of the earth’s crust. It is a metalloid with properties that are in-between or a mixture of those of metals and nonmetals. Ordinary elemental boron is a brown-black, amorphous powder. Pure boron can be made into extremely hard yellow monoclinic crystals with semiconductor properties much like silicon. Boron has two naturally occurring and stable isotopes, 11B (80.1%) and 10B (19.9%). Although the term “boron” is commonly referenced, it does not occur in nature in an elemental state. Boron combines with oxygen and other elements to form boric acid, or inorganic salts called borates. Boric acid is sometimes found in volcanic spring waters. Boron compounds, chiefly borates, are commercially important. Four borates - colemanite (see attached image), kernite (see attached image), tincal, and ulexite (see attached image) - make up 90% of the borates used by industry worldwide.
Uses: component of composite materials (boron fibers) in advanced aerospace structures; industrial catalyst for many organic reactions, such as polymerization reactions; major role in electroplating of nickel, lead and tin; inner plates of ballistic vests and for tank armor (carbon boride); permanent Neodymium-Iron-Boron (NdFeB) magnets
US Imports: Refined borax – 160,000 mt, Boric acid – 53,000 mt, Borates – 37,000 mt (2022 est.)
Import Sources (2018-21): All forms: Turkey, 90%; Bolivia, 5%; Chile, 2%; and other, 3%.
World Resources: Deposits of borates are associated with volcanic activity and arid climates, with the largest economically viable deposits located in the Mojave Desert of the US, the Alpide belt in southern Asia, and the Andean belt of South America. US deposits consist primarily of tincal, kernite, and borates contained in brines, and to a lesser extent ulexite and colemanite. About 70% of all deposits in Turkey are colemanite, primarily used in the production of heat-resistant glass. At current levels of consumption, world resources are adequate for the foreseeable future.
Substitutes: The substitution of other materials for boron is possible in detergents, enamels, insulation, and soaps. Sodium percarbonate can replace borates in detergents and requires lower temperatures to undergo hydrolysis, which is an environmental consideration. Some enamels can use other glass-producing substances, such as phosphates. Insulation substitutes include cellulose, foams, and mineral wools. In soaps, sodium and potassium salts of fatty acids can act as cleaning and emulsifying agents.
Note(s): Most of the boron products consumed in the US were manufactured domestically. Estimated boron production increased in 2022 compared with 2021 production.
Germanium (Ge/32)
Description: Germanium is mainly a byproduct of zinc ore processing. It is a hard, grayish-white element and has a metallic luster and the same crystal structure as diamond; it is also brittle, like glass. Germanium is a semiconductor, with electrical properties between those of a metal and an insulator.
Uses: polymerization catalyst for polyethylene terephthalates (PET); telecommunication fiber optics; lenses for mid- and long- wavelength infrared (IR) devices; solar cells
US Imports: Germanium metal 14 mt; Germanium dioxide 15 mt (2022 est.) The US is more than 50% import reliant for its germanium needs.
Import Sources (2018-21): Germanium metal: China, 54%; Belgium, 27%; Germany, 9%; Russia, 8%; and other, 2%.
World Resources: China was a leading global exporter of germanium in 2022. The available resources of germanium are associated with certain zinc and lead-zinc-copper sulfide ores. Substantial US reserves of recoverable germanium are contained in zinc deposits in Alaska and Tennessee. Based on an analysis of zinc concentrates, US reserves of zinc may contain as much as 2,500 tons of germanium. Because zinc concentrates are shipped globally and blended at smelters, however, the recoverable germanium in zinc reserves cannot be determined. On a global scale, as little as 3% of the germanium contained in zinc concentrates is recovered. Significant amounts of germanium are contained in ash and flue dust generated in the combustion of certain coals for power generation.
Substitutes: Silicon can be a less-expensive substitute for germanium in certain electronic applications. Some metallic compounds can be substituted in high-frequency electronics applications and in some light-emitting-diode applications. Zinc selenide and germanium glass substitute for germanium metal in infrared applications systems, but often at the expense of performance. Antimony and titanium are substitutes for use as polymerization catalysts.
Note(s): The major global end uses for germanium were electronics and solar applications, fiber-optic systems, infrared optics, and polymerization catalysts. Other uses included chemotherapy, metallurgy, and phosphors. Germanium-containing infrared optics are primarily for military use, but the demand for thermal-imaging devices that use germanium lenses increased during the past few years. Fiber-optic cable manufacturing accounted for about one-third of global germanium consumption.
Graphite / Carbon (C/6)
Description: Graphite is a form of pure carbon that normally occurs as black crystal flakes and masses. It has important properties, such as chemical inertness, thermal stability, high electrical conductivity, and lubricity (slipperiness) that make it suitable for many industrial applications. Graphite ores are classified as “amorphous” (microcrystalline), and “crystalline” (see attached image) (“flake” or “lump or chip”) based on the ore’s crystallinity, grain-size, and morphology. All graphite deposits mined today formed from metamorphism of carbonaceous sedimentary rocks, and the ore type is determined by the geologic setting.
Uses: The major uses of natural graphite were in brake linings, lubricants, powdered metals, refractory applications, and steelmaking. Steelmaking and refractory applications in metallurgy use the largest amount of produced graphite; however, emerging technology uses in large-scale fuel cell, battery, and lightweight high-strength composite applications could substantially increase world demand for graphite.
US Imports: 82,000 mt (2022 est.). The US is 100% import reliant for its graphite needs.
Import Sources (2018-21): China, 33%; Mexico, 18%; Canada, 17%; Madagascar, 10%; and other, 22%.
World Resources: US domestic resources of graphite are relatively small, but the rest of the world’s inferred resources exceed 800 million tons of recoverable graphite.
Substitutes: Synthetic graphite powder, scrap from discarded machined shapes, and calcined petroleum coke compete for use in iron and steel production. Synthetic graphite powder and secondary synthetic graphite from machining graphite shapes compete for use in battery applications. Finely ground coke with olivine is a potential competitor in foundry-facing applications. Molybdenum disulfide competes as a dry lubricant but is more sensitive to oxidizing conditions.
Note(s): In 2022, China was the world’s leading graphite producer, producing an estimated 65% of total world production. Approximately 24% of production in China was amorphous graphite and about 76% was flake. Large graphite deposits were being developed in Madagascar, northern Mozambique, Namibia, and south-central Tanzania. A graphite mine in Mozambique in a high-grade graphite deposit was reportedly the largest natural graphite mine globally. In 2022, an Australian company commissioned a graphite anode plant in Sweden, becoming the first commercial plant operating in Europe.
Helium (He/2)
Description: colorless, odorless, inert gas
Uses: magnetic resonance imaging, 30%; lifting gas, 17%; analytical and laboratory applications, 14%; welding, 9%; engineering and scientific applications, 6%; leak detection and semiconductor manufacturing, 5% each; and various other minor applications, 14%.
US imports: 8 million cubic meters (2022 est.)
Import Sources (2018-21): Qatar, 53%; Canada, 20%; Algeria, 15%; Russia, 5%; and other, 7%.
World Resources: As of year end 2021, the mean volume of recoverable helium within the known geologic natural gas reservoirs in the United States was estimated to be 8,490 million cubic meters. Helium resources of the world, exclusive of the United States, were estimated to be about 31.3 billion cubic meters. The locations and volumes of the major deposits, in billion cubic meters, are Qatar, 10.1; Algeria, 8.2; Russia, 6.8; Canada, 2.0; and China, 1.1.
Substitutes: There is no substitute for helium in cryogenic applications if temperatures below -256°C (-429°F) are required. Argon can be substituted for helium in welding, and hydrogen can be substituted for helium in some lighter-than-air applications in which the flammable nature of hydrogen is not objectionable. Hydrogen is also being investigated as a substitute for helium in deep-sea diving applications below 305 m (1,000 ft).
Natural Rubber
Description: Natural rubber is produced from rubber trees as a latex liquid. Rubber is very useful because it is waterproof, is highly elastic, and is highly resilient.
Uses: tires and inner tubes; footwear; gasket packaging and sealing; hoses and belting
US Imports: 34,885,568 mt (2021) The US is 100% import reliant for its natural rubber needs.
Import Sources (2020): Indonesia, 56%; Thailand, 23%; Cote d’Ivoire, 6%; Liberia, 3%; Malaysia, 3%; Vietnam, 3%; other, 4%
World Resources: Largest producers of natural rubber are Thailand, Indonesia, Malaysia, Vietnam, India, China, Philippines, and Nigeria.
Quartz Crystal
Description: Industrial cultured quartz crystal is electronic-grade quartz crystal that is manufactured, not mined. Cultured or synthetic quartz is produced by a hydrothermal process and is used for its unique piezoelectric properties. Used in crystal oscillators within watches and clocks, signal stabilization with radio transmitters and receivers, sensor material in extremely sensitive scales, and in Global Positioning Systems (GPS).
Uses: military radios; electronic warfare; guidance systems; radar; navigation; aviation electronics
US Imports: Piezoelectric quartz 83 mt (2022)
Import Sources (2018-21): Piezoelectric quartz - China, 90%; Japan, 3%; Russia, 2%; and other, 5%.
World Resources: Limited resources of natural quartz crystal suitable for direct electronic or optical use are available throughout the world. World dependence on these resources will continue to decline because of the increased acceptance of cultured quartz crystal as an alternative material.
Substitutes: Silicon is increasingly being used as a substitute for quartz crystal for frequency-control oscillators in electronic circuits. Other materials, such as aluminum orthophosphate (the very rare mineral berlinite), langasite, lithium niobate, and lithium tantalate, which have larger piezoelectric coupling constants, have been studied and used. The cost competitiveness of these materials, as opposed to cultured quartz crystal, is dependent on the type of application that the material is used for and the processing required.
Selenium (Se/34)
Description: Selenium is a purplish-gray nonmetal semiconductor with an unusual property: its conductivity is proportional to the intensity of light shined onto it. Also, selenium can produce electricity directly from sunlight, making it useful in solar cells.
Uses: largely consumed in metallurgy and the manufacturing of glass; electrolytic manganese (selenium dioxide); lead-acid batteries; solar cells (copper indium gallium diselenide, CIGs)
US Imports: Selenium metal 360 mt, Selenium dioxide 10 mt (2022 est.) The US is more than 50% import reliant for its selenium needs.
Import Sources (2018-21): Selenium metal: Philippines, 21%; Mexico, 15%; Germany, 13%; China,7 9%; and other, 42%. Selenium dioxide: South Korea, 79%; China, 8%; Germany, 6%; Philippines, 5%; and other, 2%.
World Resources: Reserves for selenium are based on identified copper deposits and average selenium content. Coal generally contains between 0.5 and 12 parts per million of selenium, or about 80 to 90 times the average for copper deposits. The supply of selenium is directly affected by the supply of the materials from which it is a byproduct - copper, and to a lesser extent, nickel - and it is directly affected by the number of facilities that recover selenium.
Substitutes: Silicon is the major substitute for selenium in low- and medium-voltage rectifiers. Organic pigments have been developed as substitutes for cadmium sulfoselenide pigments. Other substitutes include cerium oxide as either a colorant or decolorant in glass; tellurium in pigments and rubber; bismuth, lead, and tellurium in free machining alloys; and bismuth and tellurium in lead-free brasses. Sulfur dioxide can be used as a replacement for selenium dioxide in the production of electrolytic manganese metal, but it is not as energy efficient.
Note(s): Selenium is an essential micronutrient and is used as a human dietary supplement, a dietary supplement for livestock, and as a fertilizer additive to enrich selenium-poor soils. Selenium also is used as an active ingredient in anti-dandruff shampoos. Estimates for world consumption are as follows: metallurgy (including manganese production), 40%; glass manufacturing, 25%; agriculture, 10%; chemicals and pigments, 10%; electronics, 10%; and other uses, 5%.
Silicon (Si/14)
Description: Silicon is a hard and brittle crystalline solid with a blue-grey metallic luster; it is a tetravalent metalloid and semiconductor. Silicon carbide (SiC) is a synthetic mineral most commonly produced in electrical resistance furnaces by the Acheson process. A mixture of carbon material (usually petroleum coke) and either silica or quartz sand is reacted at high temperatures (1,700 – 2,500°C) resulting in the formation of α-SiC. Silicon carbide occurs in nature as the extremely rare mineral moissanite. Virtually all the silicon carbide sold in the world is synthetic. SiC has an outstanding hardness, only surpassed by diamond, cubic boron nitride, and boron carbide.
Uses: machining or finishing cast iron, non-ferrous metals, stone, leather and rubber; pressure blasting, lapping, grinding and polishing of hard metal alloys and non-metallic materials; slicing of silicon wafers; finishing and polishing of manufactured equipment; clean and shot peen jet rotor blades and other precision parts to increase resistance to fatigue failure
US Imports: Ferrosilicon – 200,000 mt; Silicon metal – 120,000 mt (2022 est.) The US is 45% import reliant for its silicon needs.
Import Sources (2017-20): Ferrosilicon: Russia, 40%; Canada, 14%; Brazil, 12%; Malaysia, 9%; and other, 25%. Silicon metal: : Brazil, 31%; Canada, 24%; Norway, 14%; Thailand, 7%; and other, 24%. Total: Russia, 22%; Brazil, 20%; Canada, 18%; Norway, 8%; and other, 32%.
World Resources: World and domestic resources for making silicon metal and alloys are abundant and, in most producing countries, adequate to supply world requirements for many decades. The source of the silicon is silica in various natural forms, such as quartzite. Excluding the United States, ferrosilicon accounted for over 60% of world silicon production on a silicon-content basis in 2022. The leading countries for ferrosilicon production were, in descending order on a silicon-content basis, China, Russia, and Norway. For silicon metal, the leading producers were, in descending order on a silicon-content basis, China, Brazil, and Norway. China accounted for almost 70% of total global estimated production of silicon materials in 2022. Global production of silicon materials, on a silicon-content basis, was estimated to be about 4% less than that in 2021.
Substitutes: Aluminum, silicon carbide, and silicomanganese can be substituted for ferrosilicon in some applications. Gallium arsenide and germanium are the principal substitutes for silicon in semiconductor and infrared applications.
Note(s): The main consumers of silicon metal were producers of aluminum alloys and the chemical industry. The semiconductor and solar energy industries, which manufacture chips for computers and photovoltaic cells from high-purity silicon, respectively, accounted for only a small percentage of silicon demand.
Tellurium (Te/52)
Description: Tellurium is a brittle, silver-white metalloid that appears similar to tin and is mildly toxic to people. Tellurium is primarily alloyed with steel and copper to improve machining and alloyed with bismuth for thermoelectric devices. Tellurium is sometimes found in its native form as elemental crystals, but is more often found as the tellurides of gold such as calaverite (see attached image) and krennerite, petzite, and sylvanite (see attached image). Tellurium itself, is normally extracted as a by-product of copper and lead production.
Uses: alloying additive in steel, copper, lead, and cast iron; vulcanizing agent (rubber); thermoelectric devices; as a component of the compound Cadmium-Zinc-Telluride (CdZnTe) substrates in mid- and long-wave infrared devices; metal alloys
US Imports: 50 mt (2022 est.) The US is more than 75% import reliant for its tellurium needs.
Import Sources (2018-21): Canada, 52%; Germany, 24%; China, 12%; Philippines, 8%; and other, 4%.
World Resources: Data on tellurium resources is not available. World production of tellurium was estimated to be about 580 mt in 2021. More than 90% of tellurium has been produced from anode slimes collected from electrolytic copper refining, and the remainder was derived from skimmings at lead refineries and from flue dusts and gases generated during the smelting of bismuth, copper, and lead-zinc ores. Other potential sources of tellurium include bismuth telluride and gold telluride ores.
Substitutes: Several materials can replace tellurium in most of its uses, but usually with losses in efficiency or product characteristics. Bismuth, calcium, lead, phosphorus, selenium, and sulfur can be used in place of tellurium in many free-machining steels. Several of the chemical process reactions catalyzed by tellurium can be carried out with other catalysts or by means of noncatalyzed processes. In rubber compounding, sulfur and (or) selenium can act as vulcanization agents in place of tellurium. The selenides and sulfides of niobium and tantalum can serve as electrical conducting solid lubricants in place of tellurides of those metals.
Note(s): China was the leading producer of refined tellurium in 2022 and accounted for 53% of estimated global output. Estimated end uses for tellurium in global consumption were solar power cells, 40%; thermoelectric production, 30%; metallurgy, 15%; rubber applications, 5%; and other, 10%.
Rare earth elements
Cerium (Ce/58)
Description: A very reactive iron-gray colored metal and the most abundant of the lanthanide series. Cerium averages 63 mg/kg, making it the 26th most abundant element in the earth’s crust. It is mostly used in one of its many oxide states, as the unalloyed metal is toxic and reactive.
Uses: glass manufacture additive and polishing compound; phosphors in TV screens and fluorescent lamps; chemical oxidizing agent; ceramic capacitors, semiconductors and other LCD components; wastewater treatment
US imports: Ferrocerium compounds 420 mt (2022 est.)
Import Sources (2018-21): Rare-earth compounds and metals: China, 74%; Malaysia, 8%; Estonia and Japan, 5% each; and other, 8%; Compounds and metals imported from Estonia, Japan, and Malaysia were derived from mineral concentrates and chemical intermediates produced in Australia, China, and elsewhere.
World Resources: Rare earths are relatively abundant in the Earth’s crust, but minable concentrations are less common than for most other ores. Resources are primarily in four geologic environments: carbonatites, alkaline igneous systems, ion-adsorption clay deposits, and monazite-xenotime-bearing placer deposits. Carbonatites and placer deposits are the leading sources of production of light rare-earth elements. Ion-adsorption clays are the leading source of production of heavy rare-earth elements. Bastnäsite (see attached image) and the phosphate mineral monazite (see attached) are the two largest sources of cerium and other rare-earth elements. Mountain Pass (see attached image) is the only operating rare earth elements (REE) mine in the US. It is located it the Mojave Desert of southeastern California. Bastnäsite is the dominant REE ore mineral at Mountain Pass.
Substitutes: Substitutes are available for many applications but generally are less effective.
Note(s): The estimated distribution of rare earths by end use was as follows: catalysts, 74%; ceramics and glass, 10%; metallurgical applications and alloys, 6%; polishing, 4%; and other, 6%.
Open pit rare earth element mine at Mountain Pass, California.
Dysprosium (Dy/66)
Description: A soft metal with a bright silver luster. The metal is a by-product in the commercial production of yttrium.
Uses: permanent magnets; high-intensity lighting; capacitors and chips; data storage applications; chemical reaction testing; laser materials (ceramics and specialty glass)
World resources: Global mine production was estimated to have increased to 300,000 tons of rare earth oxide (REO) equivalent in 2022. In North America, measured and indicated resources of rare earths were estimated to include 3.6 million tons in the United States and more than 14 million tons in Canada.
Erbium (Er/68)
Description: A bright, silvery metal. It belongs to the heavy rare earth elements that are less abundant in nature. Erbium occurs in nature in mixtures with other lanthanide elements. A common mineral is gadolinite. The metal is fairly stable in air and does not oxidize as rapidly as some other metals.
Uses: Yttrium-Aluminum-Garnet (YAG) laser applications; lasers used for cutting and welding; alloy additive for vanadium; activator for phosphors; fiber optic cables; erbium-doped optical fiber amplifiers (EDFAs)
World resources: Global mine production was estimated to have increased to 300,000 tons of rare earth oxide (REO) equivalent in 2022. In North America, measured and indicated resources of rare earths were estimated to include 3.6 million tons in the United States and more than 14 million tons in Canada.
Europium (Eu/63)
Description: A soft silvery metal. Europium has the second lowest melting point and the lowest density of all lanthanides. It ignites in air at 150–180°C to form europium oxide and is the most reactive of the rare earth elements. The metal is soft and quite ductile. Europium is a fission product generated in nuclear reactors. Europium is not found in nature as a free element but is found mixed with other rare earth elements.
Uses: phosphors used in display screens, TVs and fluorescent lights, ceramics and specialty glass, activator for yttrium-based phosphors in TVs and computer screens, polishing powders and magnets
World resources: Global mine production was estimated to have increased to 300,000 tons of REO equivalent in 2022. In North America, measured and indicated resources of rare earths were estimated to include 3.6 million tons in the United States and more than 14 million tons in Canada.
Gadolinium (Gd/64)
Description: A silvery white ductile metal which is classified as a light rare earth element. It is relatively stable in dry air but tarnishes in moist air. It is ferromagnetic at temperatures below 20°C and paramagnetic above this temperature.
Uses: medical services as an MRI contrast agent and in X-ray tubes, high refractive index glass or garnets, added to chromium, iron and related alloys, high power permanent magnets, lasers, radar warning receivers and radar jammers, optical lenses, optical fibers and coatings, because Gadolinium has the highest thermal neutron capture cross-section of any known element it is used to target tumors in neutron therapy
World resources: Gadolinium is produced both from monazite and bastnasite deposits.
Note(s): Gadolinium possesses unusual metallurgical properties, to the extent that as little as 1% of gadolinium can significantly improve the workability and resistance to oxidation at high temperatures of iron, chromium, and related metals. Gadolinium as a metal or a salt absorbs neutrons and is, therefore, used sometimes for shielding in neutron radiography and in nuclear reactors. It is used as a secondary, emergency shut-down measure in some nuclear reactors.
Holmium (Ho/67)
Description: A soft, malleable metal with a bright silver luster. It oxidizes rapidly in moist air and at elevated temperatures. It falls within the heavy lanthanide rare earth elements and has the strongest magnetic moment of any natural element.
Uses: strong, artificially generated magnetic fields; red/yellow colors in glass; calibration in gamma ray spectrometers; solid state Yttrium-Iron-Garnet (YIG) and Yttrium-Lithium-Fluoride (YLF) lasers
World Resources: Holmium is found as a minor component of the minerals monazite and bastnaesite. It is extracted via ion exchange and solvent extraction from ores that are processed to extract yttrium. The main producers are China, Russia, and Malaysia.
Lanthanum (La/57)
Description: A soft, silver white metal. It is rarely kept in elemental form because it quickly oxidizes in air; it burns easily when ignited. Its oxide is much more stable and is the basis for most applications that use lanthanum.
Uses: optical fibers, glasses, and lenses; ceramic capacitors, semiconductors, and other LCD and electronic components; metal alloys for nickel metal hydride batteries; fiber-optic communication systems; samarium cobalt magnets; high-strength, low-alloy steel; infrared-absorbing glass for night vision goggles
World resources: Lanthanum is found in rare earth minerals, principally monazite (25% lanthanum) and bastnaesite (38% lanthanum). The main producers are China, Russia, and Malaysia.
Note(s): Ion-exchange and solvent extraction techniques are used to isolate rare earth elements from minerals. Lanthanum metal is usually obtained by reducing the anhydrous fluoride with calcium. Nickel-metal hydride batteries use anodes made of a lanthanum-based alloys.
Lutetium (Lu/71)
Description: A silvery white metal that is relatively stable in air. It is found in very small amounts in almost all minerals containing yttrium. Commercially extracted from monazite, it is one of the most difficult metals to prepare. It is one of the rarest and the most expensive of the rare earth metals with a price about US $10,000 per kilogram. It has very few commercial applications and is used primarily in research.
Uses: high-refractive-index optical Lutetium-Aluminum-Garnet (LuAG) lenses; X-ray phosphors; specialty silicon nitride ceramic bearings; catalyst in cracking hydrocarbons in oil refineries
World resources: In common with many other lanthanides, the main source of lutetium is the mineral monazite. It is extracted, with difficulty, by reducing the anhydrous fluoride with calcium metal. The main producers are China, Russia, and Malaysia.
Neodymium (Nd/60)
Description: A soft, bright, silvery metal. It is one of the most reactive of rare earth elements and quickly oxidizes in air. The primary source of neodymium is from carbonatites and bastnaesite (see attached image), and a secondary source is in monazite. It is found in minerals such as cerite and allanite. The pure metal has limited application. A component, along with praseodymium, of didymium glass.
Uses: glass production; incandescent light bulbs; cathode ray tubes; ceramic capacitors, semiconductors, and other components for LCDs and electronics; Neodymium-Iron-Boron (NdFeB) magnets in smartphones, hard drives, other consumer electronics; gas turbine ship propulsion
World resources: The main sources of most lanthanide elements are the minerals monazite and bastnaesite. Neodymium can be extracted from these minerals by ion exchange and solvent extraction. The element can also be obtained by reducing anhydrous neodymium chloride or fluoride with calcium. The main producers are China, Russia, and Malaysia.
Note(s): Neodymium glass is used to make lasers. These are used as laser pointers, as well as in eye surgery, cosmetic surgery and for the treatment of skin cancers. Neodymium-Iron-Boron magnets, which are the strongest known type of magnets, are used when space and weight are restrictions.
Neodymium metal and neodymium oxide powder
Praseodymium (Pr/59)
Description: A soft, silvery, malleable, and ductile metal. Its average concentration in earth’s crust makes it more abundant than silver, gold, or antimony. It is too reactive to be found in native form, and pure praseodymium metal slowly develops a green oxide coating when exposed to air.
Uses: doping agent in fiber optic cables and several metal alloys; thermal resistant alloys; optical lenses, filters, and coatings; ceramic capacitors, semiconductors, and other components in LCDs and electronics; Neodymium-Iron-Boron (NdFeB) high-power magnets; alloyed with magnesium in aircraft engines; lasers
World resources: Found in nature associated with other rare earth elements. Monazite and bastnaesite are the two principal commercial sources for praseodymium production, even though it is also found in apatite, trachyte, fergusonite, and eudialyte.
Samarium (Sm/62)
Description: A bright silver metal that is reasonably stable in air. Widely distributed in nature but in trace quantities always associated with other rare earth elements. The commercial source of samarium is from carbonatites and bastnaesite. It is also found in Precambrian granite rocks, shale, and in minerals such as xenotime and basalt.
Uses: Samarium-cobalt permanent magnets used in tank navigation; present in Neodymium-Yttrium-Aluminum-Garnet (NYAG) laser glass; infrared absorption glass; optical glass
World resources: Global mine production was estimated to have increased to 300,000 tons of rare earth oxide (REO) equivalent in 2022. In North America, measured and indicated resources of rare earths were estimated to include 3.6 million tons in the United States and more than 14 million tons in Canada.
Scandium (Sc/21)
Description: Scandium is a transition metal that is silvery white, soft and light, and has historically been classified as a rare-earth element, together with yttrium and the lanthanides. It is found widely dispersed in low concentrations in many minerals, but primarily as a trace constituent of ferro magnesium minerals. Thortveitite (a Scandium-Yttrium silicate) is the only mineral containing large amounts of scandium, about 34%; unfortunately this mineral is quite rare and is not an important source of scandium.The strengthening effects of scandium on aluminum alloys were discovered in the 1970s, and its use in such alloys remains its only major application.
Uses: scandium alloy in pistol frames; electronics, light aluminum-scandium alloy for aerospace components; lasers; high-intensity lamps for landing gear; solid oxide fuel cells; ceramics
US Imports: rare earth metals including Scandium 520 mt (2022 est.). The US is 100% import reliant for its scandium needs.
Import Sources (2018-21): Although no definitive data exist listing import sources, imported material is mostly from Europe, China, Japan, and the Philippines.
World Resources: Resources of scandium are abundant. Scandium is more abundant in the earth's crust than lead. Scandium lacks affinity for the common ore-forming anions; therefore, it is widely dispersed in the lithosphere and forms solid solutions with low concentrations in more than 100 minerals. As a result of its low concentration, scandium is produced exclusively as a byproduct during processing of various ores or recovered from previously processed tailings or residues. Historically, scandium was produced as byproduct material in China (iron ore, rare earths, titanium, and zirconium), Kazakhstan (uranium), the Philippines (nickel), Russia (apatite and uranium), and Ukraine (uranium).
Substitutes: Titanium and aluminum high-strength alloys, as well as carbon-fiber materials, may substitute in high performance scandium-alloy applications. Light-emitting diodes displace mercury-vapor high-intensity lights in some industrial and residential applications. In some applications that rely on scandium’s unique properties, substitution is not possible.
Note(s): The principal uses for scandium in 2022 were in aluminum-scandium alloys and solid oxide fuel cells. Other uses for scandium included ceramics, electronics, lasers, lighting, and radioactive isotopes.
Scandium metal and Scandium oxide
Terbium (Tb/65)
Description: A silvery-grey rare earth metal that is malleable and ductile, soft enough to be cut with a knife, and relatively stable in air compared with other lanthanides. It is a fairly electropositive metal that reacts with water, evolving hydrogen gas. Terbium is contained along with other rare earth elements in many minerals, including monazite, xenotime and euxenite. Small amounts of terbium occur in bastnäsite and monazite. Due to the large volumes of bastnäsite processed relative to the ion-adsorption clays, a significant proportion of the world's terbium supply comes from bastnäsite (see attached image).
Uses: green phosphors in compact fluorescent light bulbs, LCDs, video displays and night vision goggles; additive in high-strength neodymium iron boron (NdFeB) magnets; lasers
World resources: Global mine production was estimated to have increased to 300,000 tons of rare earth oxide (REO) equivalent in 2022. In North America, measured and indicated resources of rare earths were estimated to include 3.6 million tons in the United States and more than 14 million tons in Canada.
Thulium (Tm/69)
Description: A silvery-white, lustrous metal that is soft, malleable, and ductile. Thulium is the second-rarest element after promethium. It is very difficult to separate from the other rare earth elements and, because of its scarcity and high price, there are few applications for this element.
Uses: portable X-ray devices; ceramic magnets for microwave equipment
World resources: Found in small quantities with other rare earth elements in several yttrium-rich minerals such as xenotime, gadolinite, euxenite, loparite, fergusonite, yttroparisite, and samaskite, but extracted commercially from monazite.
Note(s): The wavelength of thulium-based lasers is very efficient for superficial ablation of tissue, with minimal coagulation depth in air or in water. This feature makes thulium lasers attractive for laser-based surgery. Thulium has been used in high-temperature superconductors similarly to yttrium
Ytterbium (Yb/70)
Description: A soft, malleable, and ductile silvery metal; its concentration in the upper continental crust is ~1.96 mg/kg. Found naturally in the minerals euxenite, gadolinite, monazite and xenotime, but it is principally commercially extracted from monazite sand that contains ~0.03% Yb.
Uses: portable X-ray machines; optical glasses, crystals, and ceramics; ytterbium lasers are used to heat treat turbine blades; super alloys for jet engines; infrared lasers
World resources: Global mine production was estimated to have increased to 300,000 tons of rare earth oxide (REO) equivalent in 2022. In North America, measured and indicated resources of rare earths were estimated to include 3.6 million tons in the United States and more than 14 million tons in Canada.
Yttrium (Y/39)
Description: A soft, silver-colored metal that has similar properties to the lanthanides and is classified with the rare earth elements; its abundance in the earth’s crust is ~ 21 mg/kg, making it the 28th most abundant crustal element. Yttrium occurs with most rare earths deposits.
Uses: metallic alloy component; garnet crystals; LED phosphor for white and grey colors; optical and camera lenses; protective ceramic layers in jet engines; heat-resistant superalloys for jet engines; Yttrium-Aluminum-Garnet (YAG) and Yttrium-Iron-Garnet (YIG) laser crystals
US imports: Yttrium oxide 1,000 mt (2022 est.). The US is 100% import reliant for its yttrium needs.
Import sources (2018-21): Yttrium compounds: China, 94%; Germany, 3%; South Korea 1%; Japan, 1%; and other, 1%. Nearly all imports of yttrium metal and compounds are derived from mineral concentrates produced in China.
World Resources: Large resources of yttrium in monazite (see attached image) and xenotime (see attached image) are available worldwide in placer deposits, carbonatites, uranium ores, and weathered clay deposits (ion-adsorption ore). Additional resources of yttrium occur in apatite-magnetite-bearing rocks, deposits of niobium-tantalum minerals, non-placer monazite-bearing deposits, sedimentary phosphate deposits, and uranium ores. Global reserves of Yttrium oxide equivalent were estimated to be more than 500,000 metric tons. The leading countries for these reserves included Australia, Brazil, Canada, China, and India.
Substitutes: Substitutes for yttrium are available for some applications but generally are much less effective. In most uses, especially in electronics, lasers, and phosphors, yttrium is generally not subject to substitution by other elements. As a stabilizer in zirconia ceramics, yttrium oxide may be substituted with calcium oxide or magnesium oxide, but the substitutes generally impart lower toughness.
Note(s): China produces most of the world’s supply of yttrium from its weathered clay, ion-adsorption ore deposits in the southern provinces - primarily Fujian, Guangdong, and Jiangxi - and from a lesser number of deposits in Guangxi and Hunan Provinces. Processing occurs primarily at facilities in Guangdong, Jiangsu, and Jiangxi Provinces. Yttrium was produced from similar clay deposits in Burma (Myanmar).